How Many Grams Are in 3 Moles of Hf
The answer is 0049984147027929. 1 moles Hafnium to grams 17849 grams.
Solved Please See An Attachment For Details Course Hero
You can view more details on each measurement unit.
. 1 mole is equal to 1 moles HF or 20. We assume you are converting between moles HF and gram. M 3899 107868.
Examples include mm inch 100 kg US fluid ounce 63 10 stone 4 cubic cm metres squared grams moles feet per second and many more. How many grams Hydrogen Fluoride in 1 mol. Be notified when an answer is posted.
How many grams are in 3 moles of HF. 3 moles Hydrogen Fluoride to grams 6001903 grams. The answer is 200063432.
First we calculate the molar mass of HF and then we multiply that value by 3 moles using dimensional. 5 moles Hydrogen Fluoride to grams 10003172 grams. 1 grams HF is equal to 0049984147027929 mole.
N 5988 g 18015 gmol 3324 mol. By using moles to grams formula. Molecular weight of Hf or grams The SI base unit for amount of substance is the mole.
7 moles Hafnium to grams 124943 grams. 4 moles Hafnium to grams 71396 grams. Molecular weight of Hydrogen Fluoride or mol The molecular formula for Hydrogen Fluoride is HF.
2 See answers Advertisement Advertisement josshyaby josshyaby Awnswer. Mass 3 20. You can view more details on each measurement unit.
Molar mass Of HF 1 19 20gmol. The answer is 200063432. Mass amount molar mass.
We assume you are converting between grams Hafnium and mole. How many grams HF in 1 mol. Note that rounding errors may occur so always check the results.
5 moles Hafnium to grams 89245 grams. 4 moles Hydrogen Fluoride to grams 8002537 grams. Amount HF 3moles.
AwnswerExplanationAmount HF 3molesMolar mass Of HF 1 19 20gmolAmount massmolar massMass amount molar massMass 3 20Mass 60g. The SI base unit for amount of substance is the mole. How many grams are in 3 moles of HF.
Molecular weight of HF or grams This compound is also known as Hydrogen Fluoride. Molecular weight of Hafnium or mol The molecular formula for Hafnium is Hf. You can always use our grams to moles calculator to check the result.
M n M. Type in unit symbols abbreviations or full names for units of length area mass pressure and other types. 6 moles Hydrogen Fluoride to grams 12003806 grams.
How many moles HF in 1 grams. We assume you are converting between grams HF and mole. 3 moles Hafnium to grams 53547 grams.
Want this question answered. - 6914591 alphashahkhan4378 alphashahkhan4378 02122018 Environmental Sciences Secondary School How many grams are in. The SI base unit for amount of substance is the mole.
The answer is 00056025547649728. 2 moles Hafnium to grams 35698 grams. We assume you are converting between grams Hydrogen Fluoride and mole.
The answer is 17849. Amount massmolar mass. Advertisement Advertisement Brainly User Brainly User Answer.
1 grams Hydrogen Fluoride is equal to 0049984147027929 mole. Knowing how to convert grams to moles may be helpful in numerous chemical tasks eg finding the mole fraction of a solution. As you already know how the grams to moles conversion work find the number of moles.
The SI base unit for amount of substance is the mole. How many grams Hafnium in 1 mol. How many grams are in 3 mole of HF.
M 420577332 g. We assume you are converting between grams Hydrogen Fluoride and mole. You can also verify the results by fetching the values in.
We assume you are converting between moles Hf and gram. You can view more details on each measurement unit. There are 6003 grams of HF in 3 moles of HF.
8 moles Hafnium to grams 142792 grams. 6 moles Hafnium to grams 107094 grams. Molecular weight of HF or mol This compound is also known as Hydrogen Fluoride.
The molecular formula for Hydrogen Fluoride is HF. You can view more details on each measurement unit. How many moles Hf in 1 grams.
The answer is 200063432. 1 mole is equal to 1 moles Hf or 17849 grams. 7 moles Hydrogen Fluoride to grams 1400444 grams.
1 grams Hafnium is equal to 00056025547649728 mole. The SI base unit for amount of substance is the mole. You can view more details on each measurement unit.
9 moles Hafnium.
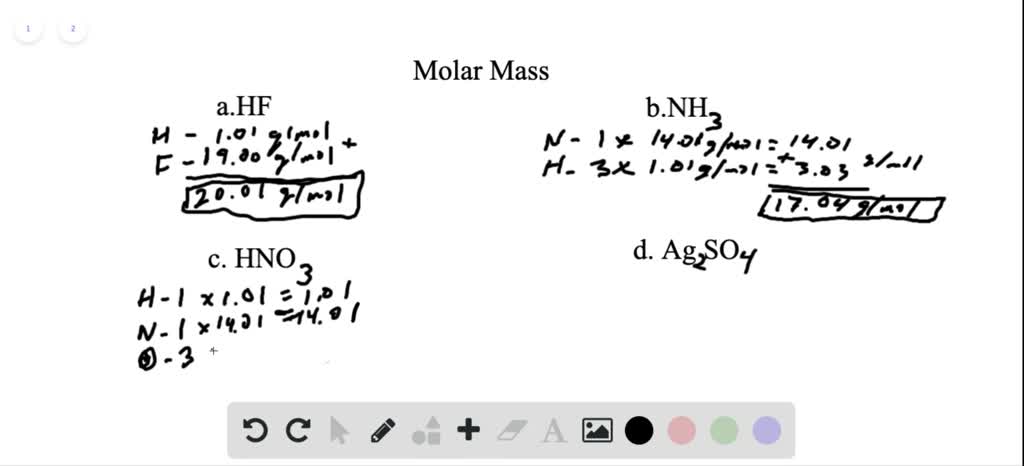
Solved Calculate The Molar Mass Of Each Of The Following Compounds A Hydrogen Fluoride Hf B Ammonia Mathrm Nh 3 C Nitric Acid Hno 3 D Silver Sulfate Mathrm Ag 2 Mathrm So 4 E Boric Acid Mathrm B Mathrm Oh 3
Solved Please See An Attachment For Details Course Hero

How Many Grams Of Hf Are Needed To React With 3 0 Moles Of Sn Brainly Com
No comments for "How Many Grams Are in 3 Moles of Hf"
Post a Comment